
Abstract The standard course of antiviral therapy for recurrent genital herpes requires administration of multiple doses of medication for 5 days. To assess the efficacy of a shorter course of antiviral therapy, patients with recurrent genital herpes simplex virus type 2 (HSV-2) infection were enrolled in a randomized, double-blind, placebo-controlled study of acyclovir (800 mg given by mouth 3 times per day [t.i.d.]) for 2 days. Of 131 people enrolled in the study, 84 (51 women and 33 men) were observed for ⩾1 recurrence and 65 were observed for 2 recurrences, for which the patient was administered the same study drug (acyclovir or placebo). Acyclovir therapy (800 mg given by mouth t.i.d. For 2 days) significantly reduced the duration of lesions (median for acyclovir versus placebo, 4 days versus 6 days; P =.001), episode (4 days versus 6 days; P.
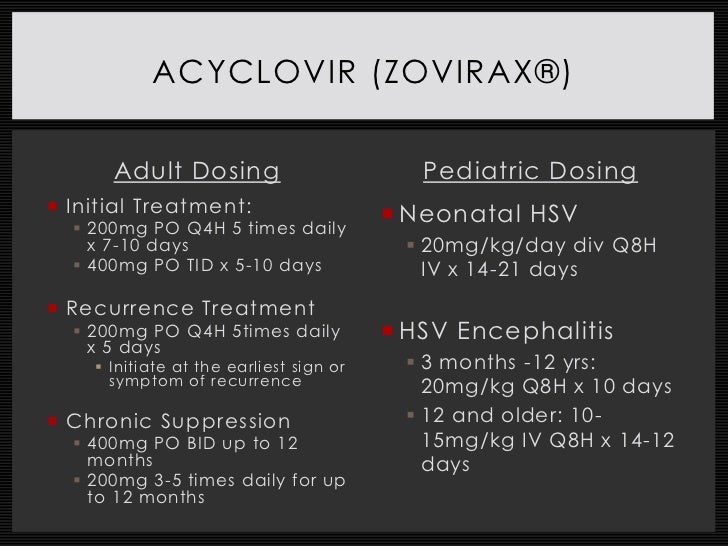
Acyclovir is a synthetic purine nucleoside analogue with inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV). Acyclovir inhibits viral DNA synthesis and must be phosphorylated intracellularly to be active. Although lesions caused by herpes simplex virus (HSV) subtypes HSV-1 and HSV-2 differ in rates of recurrence and subclinical shedding, they are treated with the same antiviral regimens, based on the site of infection. Genital herpes First episode Acyclovir 200 mg PO 5 times daily for 7-10 days or Acyclovir 400 mg PO TID for 7-10 days or F.
Antiviral therapy for recurrent genital herpes decreases the duration of lesions, discomfort, and viral shedding []. To date, such regimens require multiple daily doses administered for 5 days.
This dosing pattern was based on the administration of therapy until clinical healing of lesions was noted. However, the duration of viral replication in most lesions caused by recurrent infection in immunocompetent people is short. Therefore, we undertook a study to evaluate whether a shorter regimen of antiviral medication would also decrease the duration of a recurrent genital herpes episode. Patients and Methods Participants and study protocols. Healthy adult men and women with recurrent genital herpes simplex virus type 2 (HSV-2) infection were recruited at the University of Washington Virology Research Clinic (Seattle). Entry criteria included a history of ⩾3 recurrences in the 12 months before study entry.
Potential subjects who had been receiving suppressive antiviral therapy were required to have had a history of ⩾6 recurrences in the year before initiating suppressive therapy, and ⩾2 recurrences within 3 months of discontinuing suppressive therapy. The trial was randomized, double blind, and placebo controlled. Patients who met the entry criteria were randomized in a 1: 1 ratio to receive either acyclovir (800 mg given by mouth [po] t.i.d. Hp p2015d driver. ; the acyclovir group) or matching placebo (the placebo group) for 2 days.
Zovirax For Suppression Dosage
The same medication was dispensed for the first and second recurrence during the study. This study design was chosen so that the mean of the results from both recurrences could be used as a unit of analysis. This allowed us to recruit fewer patients than would have been required had each person been observed through only 1 recurrence. For each episode, each participant was given 3 doses of study medication and a diary card.

 0 kommentar(er)
0 kommentar(er)
